- A+
酯化反应最简单的形式是:

也是最常用的制备酯的方法。
反应速度一般反应很慢,在常温不能觉察;在回流温度也极其缓慢, 不能用于制备,必须加入催化剂加速它的进行。催化剂中最常用的是酸,如硫酸、盐酸等。如果有机酸本身酸性较强,如甲酸、草酸等,以及氨基酸的盐酸盐等,酯化时可以不加无机酸。
酯化反应是可逆反应。酯化时要把缩合所形成的水不断除去,以提高酯的产率。除去水的方法有物理方法和化学方法两类。
物理方法可用恒沸蒸馏法,即在反应系统中加入和水不相混溶的溶剂,如苯、甲苯、二甲苯、四氯化碳、氯仿等。苯:乙醇:水的成分比为 74.1:18.5:7.4时可形成三组分最低共沸液,沸点为 64.8℃;四氯化碳:乙醇:水的成分比为10:65:25 时可形成三组分最低共沸液,沸点为 61℃;
化学除水方法可以用无机盐类,如硫酸铜,它能与水形成水合晶体,但效果不甚好。硫酸和盐酸(实际上是无水氯化氢气体)是催化剂,同时也是除水剂。有效的除水剂如乙酰氯、亚硫酰氯、氯磺酸等,另外如碳二酰亚胺(R-N=C=N-R)是极好的除水剂,可在室温下酯化脱水。三氟化硼和它的乙醚复合物则既是催化剂又是除水剂,其他的还有利用吡啶衍生物、苯并三唑衍生物等。
酯化速度,在醇方面伯醇>仲醇>叔醇,叔醇的酯化一般要比脱水的速度要慢,因此用通常的酯化方法得到的是烯而不是酯;在酸方面,脂肪族酸>芳香族酸。
羧酸酯类化合物的合成
依羧酸和醇的性质可采用以下方法制取:①羧酸和醇脱水酯化;②羧酸盐和卤素作用;③羧酸盐和硫酸酯、磺酸酯作用;④酸酐和醇、酚反应;⑤酰氯和醇、酚反应;⑥羧酸酯和醇、酚酯交换反应;⑦腈的醇解反应;
硫酸作催化剂的酯化反应示例:
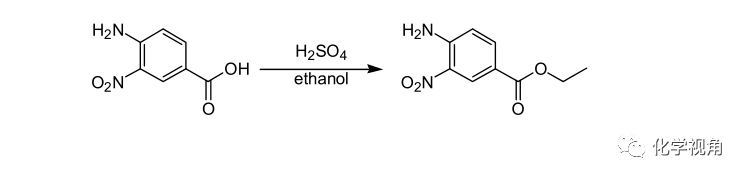
4-Amino-3-nitrobenzoic acid (200.0 g) was suspended in ethanol (1030 g). Conc.sulfuric acid (120.0 g) was added dropwise to the mixture over 0.5 h. After the dropwise addition, the mixture was refluxed under heating for 15 h. A part (422 mL) of ethanol was distilled away at atmospheric pressure, and water (660 mL) was added
dropwise to allow precipitation of crystals. To this suspension were added 25% aqueous sodium hydroxide solution (50 mL) and then 2.5% aqueous sodium hydroxide solution (262 mL), and pH was adjusted to 6.5. The precipitated crystals were collected by filtration, washed successively with ethanol (531 mL) and water (1069 mL), and dried under reduced pressure to give ethyl 4-amino-3-nitrobenzoate (200.6 g)。
盐酸(氯化氢)作催化剂的酯化反应示例:
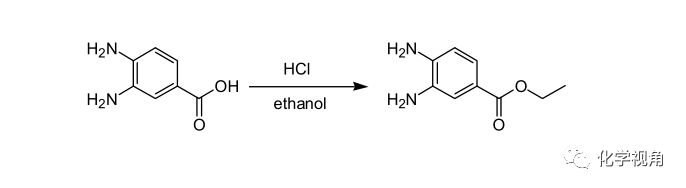
3,4-Diaminobenzoic acid (152 g, 1.0 mol) was stirred with ethanol (2000 mL) in a Morton flask. Hydrogen chloride gas was passed through the stirred suspension for about 2 h. As the gas was absorbed, the slurry became gel-like in character. Ethanol (500 ml.) was added to the reaction mixture to disperse the gel. The reaction mixture was refluxed 24 h. The mixture was filtered and the filtrate was evaporated to dryness in vacuo. The filter cake and the filtrate residue were dissolved in water (9 L). The aqueous solution was made basic by the addition of solid sodium carbonate. The product precipitated from the basic solution. The material was filtered and dried to yield ethyl 3,4-diaminobenzoate (130 g, 72.14%)。
亚硫酰氯作催化剂的酯化反应示例
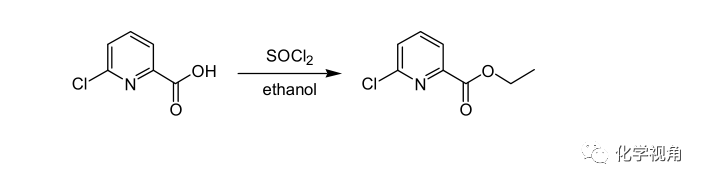
To a suspension of 6-chloropyridine-2-carboxylic acid (8.3 g, 0.0525 mol) in dioxane 25 mL), thionyl chloride (9.4 mL, 0.13 mol) was added and the resulting mixture was stirred at 70 ℃ for 4 h. The reaction mixture was concentrated in vacuo and a mixture of dioxane (8.3 mL) and ethanol (16.6 mL) was added. The reaction mixture was heated to 70 ℃ for 2 h, triethylamine (8.3 mL), ethanol (4.1 mL) and water (8.3 mL)were added and the reaction mixture was again concentrated. The residue was distributed between diethyl ether (28 mL) and water (18 mL) and the phases were separated. The aqueous layer was extracted with diethyl ether (30 mL) and the combined organic layers were dried over MgSO 4 , filtered and evaporated in vacuo to afford 6-chloropyridine-2-carboxylic acid ethyl ester (8.82 g, 91%) as oil
乙酰氯作催化剂的酯化反应示例:

Acetyl chloride (15.3 mmol) was added dropwise to ethanol (50 mL) at 0℃. After 30 min, the acid (7.69 mmol) was added and the reaction mixture was heated at reflux for 15 h. The reaction mixture was concentrated and the residue was partitioned between dichloromethane (20 mL) and saturated sodium bicarbonate (10 mL). The aqueous layer was further extracted with dichloromethane (20 mL × 2) and the combined organic layers were dried (magnesium sulfate) and concentrated to provide the ester in 94% yield as brown oil。
对甲苯磺酸作催化剂的酯化反应示例:
对甲苯磺酸作酯化催化剂只比硫酸稍弱,仍是一个强酸,其用量与使用硫酸相等分子,其作用相等或相近,使用对甲苯磺酸作为酯化催化剂主要是为了避免硫酸带来的焦化和其他副反应。
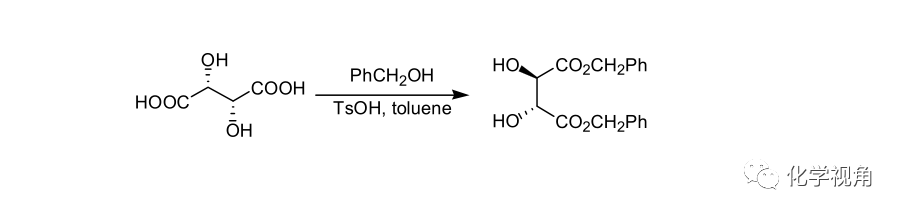
A 300-mL, one-necked, round-bottomed flask was equipped with a magnetic stirrer, Dean-Stark trap, and a reflux condenser. The flask was charged with 3.0 g (20 mmol) of L-(+)-tartaric acid, 6.5 g (60 mmol) of benzyl alcohol, 47.5 mg (0.25 mmol) of p-toluenesulfonic acid monohydrate and 40 mL of toluene. The mixture was heated under reflux in an oil bath (about 130 ℃ ) for 13 h. During this period the theoretical amount of water (0.62 mL) was collected. The mixture was allowed to cool to ambient temperature, diluted with ether, and poured into 50 mL of aqueous, saturated sodium bicarbonate. The organic phase was separated and the aqueous phase was extracted twice with 20 mL of ether. The combined organic phases were dried over sodium sulfate. The solvent was removed with a rotary evaporator, and the resulting crude product was triturated with hexane-ether (20:1, 210 mL) to give white crystals of (−)-dibenzyl tartrate. The precipitate was collected by filtration and washed with hexane-ether (20:1). The filtrate was further concentrated to give a second crop. The total yield was 6.2 g (94%), mp 49–50 ℃。
吡啶衍生物作除水剂的酯化反应示例:
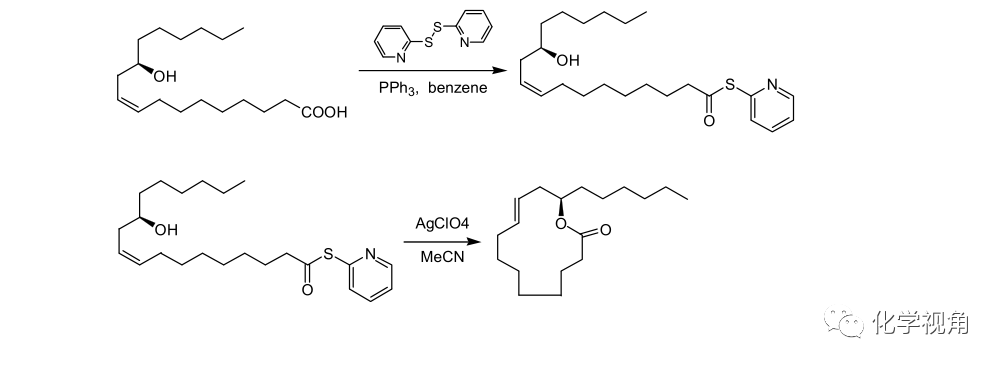
In a dry, stoppered, 10-mL flask containing a magnetic stirring bar were placed 360mg (1.2 mmol) of ricinelaidic acid, 2,2'-dipyridyl disulfide (308 mg, 1.4 mmol),benzene (1 mL), and 367 mg (1.4 mmol) of triphenylphosphine. The mixture was stirred for 30 min. The resulting slurry was then dissolved in dry acetonitrile (55 mL). B. Ricinelaidic acid lactone
Dry acetonitrile (100 mL), 3.5 mL of 1 M silver perchlorate in toluene and a magnetic stirring bar were placed in a 500-mL flask equipped with a reflux condenser that carries a Hershberg dropping funnel. The solution was heated in an oil bath so that the boiling acetonitrile returns from the condenser at the rate of 5–10 drops per second. Then the acetonitrile solution of the ricinelaidic acid S-(2-pyridyl)carbothioate was added dropwise during 1 h through the condenser to the magnetically stirred refluxing silver perchlorate solution. The slightly turbid mixture was boiled for an additional 15 min and the solvent was removed under reduced pressure in a rotary evaporator. The residue was diluted with 30 mL of 0.5 M potassium cyanide solution and the mixture containing suspended solids was extracted with three 50-mL portions of benzene. The benzene extracts were washed with 30 mL of water, dried with anhydrous magnesium sulfate, and filtered, and the solvent was removed under reduced pressure. Crude product was obtained as an oil (710 mg). It can be purified by chromatography on 40g of silica gel with benzene as eluant. Fractions of 10 mL were collected at 30 min intervals. Fractions 7–19 contain 283–296 mg (84–88%) of ricinelaidic acid lactone。
苯并三唑衍生物作除水剂的酯化反应示例:
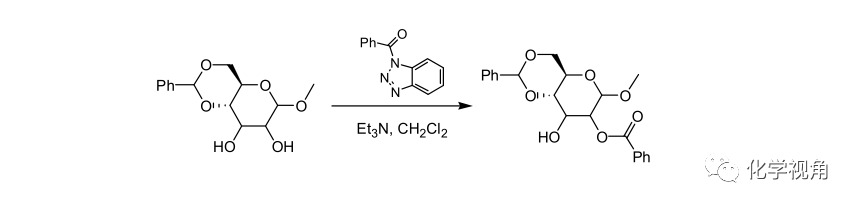
Triethylamine (305ul, 2.2 mmol) was added at room temperature to a stirred solution of 1-phenyl-1, 2-ethanediol (280 mg, 2.0 mmol) and 1-(benzolyoxy)benzotriazole (503 mg, 2.1 mmol) in CH 2 Cl 2 (8 mL). The reaction mixture was stirred at room temperature for 24 h, diluted with CH 2 Cl 2 (30 mL), washed with saturated NaHCO 3 solution (20 mL), dried over MgSO 4 , and evaporated to dryness. The crude product was subjected to silica gel column chromatography with hexane/ EtOAc (6:1) mixture as an eluent o afford the dibenzoate (34 mg, 5%), the primary monobenzoate (390 mg,83%), and the secondary nonobenzoate (42 mg, 9%)。


目前评论: